
Theropod dinosaurs and birds linked by 'breathing' design
Alec MacAndrew
Introduction
The hypothesis that modern birds are the descendants of a group of small dinosaurs, called dromaeosaurs, part of a bipedal group called theropods, has become increasingly accepted by the scientific community, to the point where it is very close to being a scientific consensus (there is a tiny and shrinking minority of scientists who disagree). Several important finds, many from China, have been made in the last few years (1) - (9) which support this hypothesis. Fossils have been found which demonstrate the evolution of feathers and the anatomical structures necessary for flight.
The evidence has persuaded almost the entire scientific community that birds evolved from theropod dinosaurs. There is, however, one aspect of bird physiology that has been a puzzle since the theory of dinosaur origins of birds was proposed. The fact is that birds have a respiratory (breathing) system unlike that of almost all other tetrapods (tetrapods include all mammals, reptiles, amphibians and birds). The puzzle centres on the fact that birds seem to have a breathing system different from all other living tetrapods without any antecedent - this is a situation that creationists have, unsurprisingly, attempted to exploit. Now, however, there is strong evidence that theropod dinosaurs that predated the emergence of birds in the record had pulmonary systems like modern bird breathing systems. This means that the flow-through lung is not unique to birds, but was present in theropod dinosaurs before the evolution and emergence of birds.
The avian flow-through lung
All tetrapods (mammals, reptiles, amphibians, crocodilians) apart from birds have a pair of lungs that operate on the bellows principle. That is, a pair of lungs that inhale and exhale air by volume changes associated with respiratory movements of the diaphragm and rib cage. The volume of the lungs are increased by inspiratory effort drawing oxygen-rich air into the lung; and are decreased to expel air partially depleted of oxygen and carrying waste carbon dioxide, from the lungs.
Birds, however, have an entirely different respiratory system. Birds breathe using a respiratory system that consists of a pair of lungs and a number of separate air sacs that take up some considerable space in the body cavity of the bird. Most birds have nine air sacs. The air sacs are connected to the lungs in such a way that when a bird breathes air is first drawn into the posterior air sacs. It then flows though the lungs in a single direction into the anterior air sacs through a fine set of 'air capillaries' (the parabronchi) in the lung. The air capillaries are closely surrounded by blood capillaries and this is where the exchange of oxygen and carbon dioxide takes place. The air sacs and lungs are arranged in such a way that air flows in the same direction through the lungs whether the bird is inhaling or exhaling (uni-directional flow-through). Unlike mammals and other tetrapods, the avian lungs remain the same volume during breathing - the air is pumped by changes in the volume of the air sacs not the lungs. The bird skeleton is highly pneumatized - that means that there are large air spaces in the bones and vertebrae of birds which connect with the air sacs.
Go to reference 10 for a detailed explanation of the avian respiratory system.
Recent finding
A recent paper in Nature (11), shows that theropod dinosaurs have vertebrae pneumatized in a way that is very similar to modern birds. The authors have investigated the well preserved fossil of a theropod dinosaur called Majungatholus atopus and have found that the vertebrae possess very close similaritiies in pneumaticity compared with an extant bird (the sarus crane). See fig 1 below.
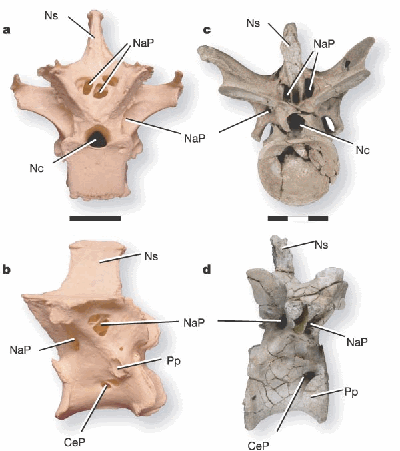
However, the similarity between the pneumatic features of theropod dinosaurs and modern birds was already known through a number of studies (12). So what does this new study indicate that we didn't know before? Detailed analysis of the individual vertebrae and ribs reveal a pattern of pneumaticity that is entirely consistent with the pattern in living birds - that is, the cervical air sac connect to vertebrae and ribs in the neck region of the spine, and in the thoracic vertebrae nearest the head; the abdominal air sac connects with the tail and sacrum vertebrae and the thoracic vertebrae nearest the tail; and the lung itself connects with the mid-thoracic vertebrae. This pattern is the same in all birds and is exactly what is found by detailed analysis of the vertebrae of M atopus. So it is not the discovery of pneumatised vertebrae in this fossil that is new, but the fact the pattern of pneumatisation is found to be the same as in living birds and consistent with a uni-directional flow-through breathing system. This situation is consistent for all known non-avian theropods, suggesting that it is a derived characteristic of the first theropods and spread throughout the entire clad of theropods including modern birds
Furthermore, O'Connor and Claessens point out that in order for either uni-directional or bi-directional flow-through ventilation to work, the tail end of the abdominal cavity has to change volume more than the head of the cavity. Indeed that is the arrangement in birds, and analysis of the skeleton of theropods shows that they possess the appropriate characteristics in the articulation of the ribs with the vertebrae to show that the tail end of the trunk can change volume mure than the head end, just as in birds. Indeed air sacs and associated features at the tail end of the abdominal cavity are known to have developed in chameleons, snakes and certain types of lizard and this indicates that the tail end of the lung in the entire sauropsid (a group that includes birds and most reptiles and dinosaurs) is able to develop air sacs and invade the tail end of the skeleton.
So, this recent study has shown that non-avian theropod dinosaurs had the necessary anatomy for flow-through ventilation similar to extant birds and, that in the evolution of the flow-through system, the tail end air sacs likely developed before those at the head end of the trunk.
Creationist response
Carl Wieland of 'Answers in Genesis' has written a response to this paper (13), that is generally reasonably restrained, but utterly fallacious. He correctly points out that this analysis does not, in itself, resolve the issue of the steps by which a bellows type lung evolved into the avian flow-through system. His discussion is, however fundamentally flawed in one important respect: his main objection to the evolution of an avian system from a bellows system is that he cannot see how it could happen. This of course is the old canard (a term that is peculiarly well suited to this subject!) of the argument from personal incredulity. Carl cannot conceive of a pathway by which the avian lung could evolve from a bellows arrangement, so of course, in his mind, it cannot have happened. This was the original design argument used by William Paley. It was intellectual gruel then, and it is intellectual gruel now. Carl would have us believe that there is an 'in-principle' barrier to the evolution of 'flow-through' ventilation. (I think Carl means unidirectional flow-through ventilation - he doesn't seem to recognise the distinction between unidirectional and bidirectional flow-through ventilation). Of course, there is no such 'in-principle' barrier and one can think of very obvious routes by which the avian system could develop from a bellows arrangement. The first obvious step in this process, the development of a bidirectional flow-through system with air sacs positioned beyond the lung in the tail end of the trunk is strongly supported by this study. Carl asks 'How could any creature breathe while the in-between stages were evolving, while air was not yet flowing through but no longer going in and out? The answer to this is obvious: it breathes by air flowing thriough the lung bidirectionally - in other words it flows through and goes in and out.
I wonder whether Carl realises that, even in modern birds, there is a mixture of unidirectional flow through the so-called palaeopulmonic bronchi and bidirectional flow through the so-called neopulmonic bronchi.
Carl also claims to know clearly what constitutes the same and what constitutes different biblical 'kinds'. If so, he is the first man on earth to do so, as no creationist has ever defined a 'kind' in a self-consistent way.
The fact is that not only is the evidence very strong that birds evolved from theropod dinosaurs, but there is no objection 'in principle' to development of the avian respiratory system.
2. Ji, Q., Currie, P. J., Ji, S. & Norell, M. A. Two feathered dinosaurs from northeastern China. Nature 393, 753-761 (1998).
3. Xu, X., Tang, Z. & Wang, X. A therizinosaurid dinosaur with integumentary structures from China. Nature 399, 350-354 (1999).
4.Xu, X., Wang, X. & Wu, X. A dromaeosaurid dinosaur with a filamentous integument from the Yixian Formation of China. Nature 401, 262-266, 1999).
5.Xu, X., Zhou, Z. & Wang, X. The smallest known non-avian theropod dinosaur. Nature 408, 705-708 (2000).
6.Chen, P.-J., Dong, Z.-M. & Zhen, S.-N. An exceptionally well preserved theropod dinosaur from the Yixian Formation of China.Nature 391, 147-152 (1998).
7.Xu, X., Zhou, Z.-H.& Prum, R. O., Branched integumental structures in Sinornithosaurus and the origin of feathers, Nature 410, 200-204 (2001)
8. Norell*, QIANG Ji Q, Gao*, Yuan, Zhao and Wang. 'Modern' feathers on a non-avian dinosaur, Nature 416, 36 - 37 (2002)
9. Xu et al, Four winged dinosaurs from China, Nature 421, 335 - 340 (2003)
10. Go here for more information on the anatomy and physiology of avian lungs
11. O'Connor and Claessens, Basic avian pulmonary design and flow-through ventilation oin non-avian theropod dinosaurs, Nature 436, 253 - 256 (2005)
12. Serono, The Evolution of dinosaurs, Science 284, 2137 - 2147 (1999)
13 Wieland, Dinos breathed like birds?, available here
© 2005