![]()
(i)
The purpose of this essay is to show why uranium lead dating justifies the belief of YEC
The amount of 206Pb present in a rock (206Pbt) is the amount of 206Pb present during solidification (206Pbo) plus the amount of 238U present initially during solidification (238Uo) which decayed subsequently to 206Pb. Let l238 denote the decay constant of 238U. 238Ut denotes the amount of 238U present today.
|
|
|
(i) |
|---|
(Through out this essay, the subscript t refers to the measured quantity today, while the subscript o refers to the quantity present during solidification.)
204Pb does not have any parent atom. This implies that the amount of 204Pb present in a rock has remained constant since crystallization. Dividing (i) by 204Pb, we obtain
|
|
|
(ii) |
|---|
A new variable with the superscript X is defined to denote the radiogenic lead formed since the rock solidified, 206PbX
|
|
|
(iii) |
Plugging (iii) into (ii), and rearranging, we obtain
|
|
|
(iv) |
Similarly, for the decay chain of 235U, we obtain
|
|
|
(v) |
The concordia curve is obtained by
plotting the values of exp^(![]() 238*t)-1 on the X-axis and the values of exp^(
238*t)-1 on the X-axis and the values of exp^(![]() 235*t)-1 on the Y-axis.
235*t)-1 on the Y-axis.
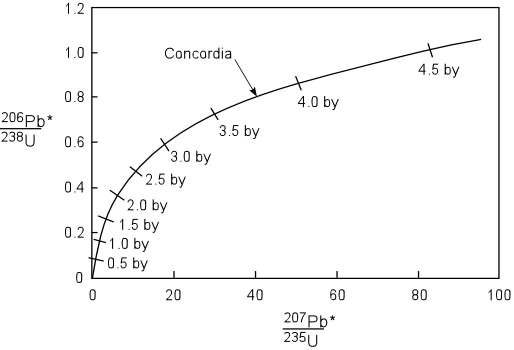
To check whether a sample of igneous rock falls on the concordia curve, a geologist first needs to determine (206Pb/204Pb)o. If molten lava flows through a lead mining site which does not contain any uranium impurity, the ratio of (206Pb/204Pb)o would be approximately 17. If lava flows through a uranium mining site containing no primordial lead, this ratio would be infinite since uranium does not decay to 204Pb. And when this magma solidifies, whatever radiogenic lead produced while the magma flowed would be preserved in the rock.
Therefore the value of (206Pb/204Pb)o can potentially have any value between 17 and infinity. In situations where this ratio cannot be determined, this method is useless.
Often there would be many samples which fall on a straight line below and above the concordia curve. Instead of admitting that the value used for (206Pb/204Pb)o could be wrong, one could make the hypothesis that there has been lead loss, uranium loss, lead addition and uranium addition to the rock after it solidified.
An example of a discordia plot (Nature Vol 366) can be found at
http://groups.yahoo.com/group/OriginsTalk/files/U-Pb%20date%20KT%201.bmp
http://groups.yahoo.com/group/OriginsTalk/files/U-Pb%20date%20KT%202.bmp
The author, in his letter to Nature interpreted the discordia line to be caused by the impact of meteorites on zircon crystal. Samples marked CH8, CH9, CH10 and CH11 when dated by the decay of 238U gave results in the range of 63 million to 73 million years. These samples are interpreted as follows: When the asteroids hit these crystals about 65 million years ago, all 206Pb was “squeezed out”, thus resetting the crystal. Thus the current 206Pb/238U ratio represents the date for the rock's last metamorphoses.
Geologists had concluded that the KT layer was 65 million years old long before radiometry was discovered. To preserve conventional wisdom, we must also throw away rocks which contain vary little radiogenic lead. The author of the above link wrote, “Data for sample C5 and C6 are imprecise due to a very low radiogenic content and are omitted for clarity.”
Zircon is known to be a very hard crystal and is resistant to weathering. Many geologists from around the world have confirmed experimentally that zircon retains its composition in extreme conditions. Even if it wasn't resistant to asteroids, it is highly unlikely that any natural and random impact can remove 206Pb selectively. If geologists want to justify that those samples were reset, they must first prove that powerful impacts can indeed reset zircon.
The straight line obtained in the discordia plot can be understood by considering the following analogy. A mixture of oil and water is solidified. Assume that carbon consists of 12C and 13C and oxygen consists of 16O and 18O. We randomly select small equal volume portions of this solidified mass for analysis. The value of 12C/16O in these samples is taken as the abscissa and the value of 13C/18O is taken as the ordinate. This plot would fall on a straight line. At locations where there was a tiny oil droplet, we would obtain a point close to the origin.
The straight line obtained in discordia dating has nothing to do with radiometry and lead loss. This fact is proven by the fact that non-radiometrically related species C and O also can produce a similar plot.
If we start with the assumption that a rock contained no radiogenic lead to begin with, the age calculated by this method would be an upper bound for the true age of the rock. This is because we do not know whether there was lead present when the rock solidified initially. The presence of 204Pb in a rock is proof that there was lead present when the rock solidified.
To determine the formation date of a particular geologic strata, a geologist should not omit rocks containing very little radiogenic lead, as these are the most important samples. These samples represent an upper bound for the formation of that strata.
Gentry realized this while dating coal and wrote, “To obtain 238U/206Pb ratios that more accurately reflect the amount of lead from in situ U decay, a search was made for sites with even higher ratios, for such areas possibly contained negligible amounts of extraneous Pb.” Gentry found samples with the 238U/206Pb ratio as high as 4*104 and 6*104. Ratios as high as this indicate that the formation of coal beds took place less than 100k years ago.
Coal beds are deeper than the KT layer. Logically speaking, the KT layer could not have been formed earlier than coal beds. Yet a comparison of 238U/206Pb ratios in these 2 strata indicate that coal beds were formed more recently. One possible explanation is that as magma flowed upwards, uranium being heavier would have sunk more easily than lead.
http://www.geocities.com/peaceharris/u238/sc194pg315.gif
http://www.geocities.com/peaceharris/u238/sc194pg316.gif
http://www.geocities.com/peaceharris/u238/sc194pg317.gif